Ru,N-Codoped Carbon Outperforms Platinum Toward Hydrogen Evolution Reaction In Alkaline Media By Atomically Dispersed Ruthenium
Background
Hydrogen evolution reaction is an important process in electrochemical energy technologies. In practice, room-temperature water electrolysis can be performed in both acidic and alkaline electrolytes. Platinum-based nanoparticles generally serve as the catalysts of choice.
Typically, these reactions are carried out under acidic conditions. However, the high cost of proton exchange membranes as well as the sluggish electron-transfer kinetics of oxygen evolution reaction under acidic conditions have limited the applications of acidic water electrolyzers.
Such issues can be mitigated when the reactions are carried out in under alkaline conditions. That said, HER under alkaline conditions comes with a significant disadvantage: HER electron-transfer kinetics about two orders of magnitude lower than that of acidic conditions with Pt catalysts. Either Pt catalysts need to be improved to better perform HER under alkaline conditions, or alternative catalysts that work better in alkaline conditions need to be developed.
Technology Description
Ruthenium has emerged as a more abundant HER catalyst with a lower cost than Pt. So the invention involves a carbon nanocomposite co-doped with individual ruthenium atoms as well as nitrogen.
Experimental results showed HER performance superior to Pt-C in alkaline media, particularly from the contribution of Ru atoms coordinated to C and N atoms, indicating that atomic Ru should be maximized relative to Ru nanoparticles.
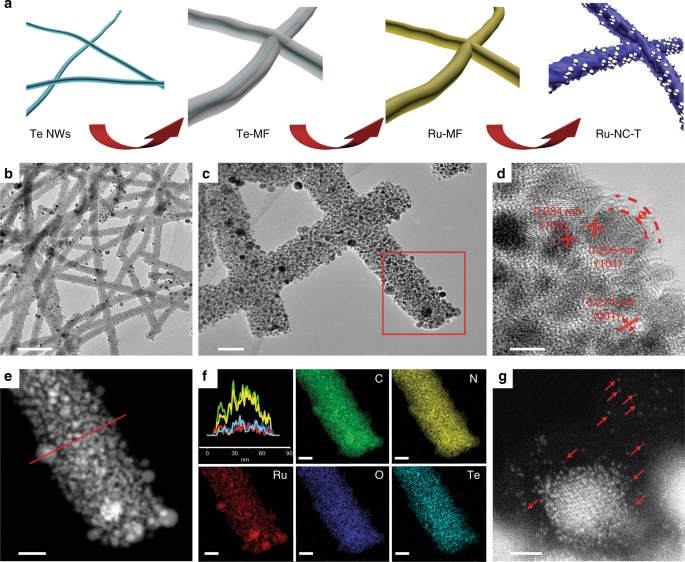
Schematic of sample preparation and transmission electron microscopic (TEM) studies. a Synthetic procedure of the Ru-NC-T samples. Te NWs denotes tellurium nanowires, Te@MF refers to tellurium nanowires with a melamine–formaldehyde resin shell, Ru-MF indicates the incorporation of ruthenium precursors into Te@MF, and Ru-NC-T signifies Ru,N-codoped carbon prepared by pyrolysis of the Ru-MF nanowires at different temperatures. b–d Representative TEM images of Ru-NC-800 at different magnifications. Scale bars are b 500 nm, c 50 nm, and d 5 nm. e High-angle annular dark-field scanning TEM image of the red area of c. The scale bar is 20 nm. f Cross-sectional elemental distributions by line scans along the red line in e. The colors of the elemental maps of C, N, O, Te, and Ru correspond to those in the line scan spectra. Scale bars are all 20 nm. g A zoom-in of e, where red arrows signify ruthenium single atoms. The scale bar is 1 nm
Applications
Hydrogen generation by electrolysis
Hydrogen generation by electrolysis under alkaline conditions
Advantages
Greater efficiency of hydrogen evolution reaction under alkaline conditions than commercially available Pt-C.
Intellectual Property Information
| Country | Type | Number | Dated | Case |
| United States Of America | Published Application | 20210355588 | 11/18/2021 | 2019-182 |
Related Materials
Contact
- Jeff M. Jackson
- jjackso6@ucsc.edu
- tel: View Phone Number.
Inventors
- Chen, Shaowei
- Guo, Lin
- Lu, Bingzhang
- Lu, Jia
- Peng, Yi
Other Information
Keywords
Hydrogen Evolution Reaction - Alkaline, Hydrogen Generation by Electrolysis - Alkaline, Clean Hydrogen Production, Hydrogen Catalysts, Hydrogen Catalysts - Alternatives to Platinum, Hydrogen Catalysts Ruthenium, Hydrogen Catalysts Atomic Ruthenium
